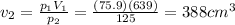Answer:
Step-by-step explanation:
For this problem, we can use Boyle's law, which states that for a gas at constant temperature, the product between pressure and volume remains constant:
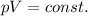
which can also be rewritten as
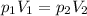
In our case, we have:
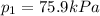 is the initial pressure
is the initial pressure
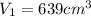 is the initial volume
is the initial volume
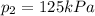 is the final pressure
is the final pressure
Solving for V2, we find the final volume: