Answer:
There are 0.93 g of glucose in 100 mL of the final solution
Step-by-step explanation:
In the first solution, the concentration of glucose (in g/L) is:
15.5 g / 0.100 L = 155 g/L
Then a 30.0 mL sample of this solution was taken and diluted to 0.500 L.
- 30.0 mL equals 0.030 L (Because 30.0 mL ÷ 1000 = 0.030 L)
The concentration of the second solution is:
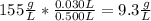
So in 1 L of the second solution there are 9.3 g of glucose, in 100 mL (or 0.1 L) there would be:
1 L --------- 9.3 g
0.1 L--------- Xg
Xg = 9.3 g * 0.1 L / 1 L = 0.93 g