Answer:
![T_(c):[Kr]5s^(2)4d^(5)](https://img.qammunity.org/2020/formulas/chemistry/college/z5zua3wfifhntama100iry76q91q03uo94.png)
Step-by-step explanation:
1. Take in account that the atomic number of the Technetium is 43.
2. Find the electronic configuration of the Technetium.
As the Technetium has 43 electrons, its electronic configuration will be:
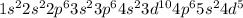
3. And the answer is expressed in terms of the electronic configuration of Kripton which has 36 electrons, so the electronic configuration of Kripton is:
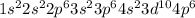
4. Therefore, the electronic configuration configuration of the Technetium is:
![[Kr]5s^(2)4d^(5)](https://img.qammunity.org/2020/formulas/chemistry/college/f5phi3co806fqvvsnywt84a01iqbza5ksm.png)