Answer: The mass or zinc reacted is 0.624 grams.
Step-by-step explanation:
We are given:
Total pressure = 1.032 atm
Vapor pressure of water = 32 torr = 0.042 atm (Conversion factor: 1 atm = 760 torr)
To calculate partial pressure of hydrogen gas, we use the equation:
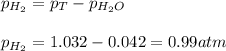
To calculate the number of moles of hydrogen gas, we use the equation given by ideal gas follows:

where,
P = pressure of hydrogen gas = 0.99 atm
V = Volume of hydrogen gas = 240. mL = 0.240 L (Conversion factor: 1 L = 1000 mL)
T = Temperature of hydrogen gas =
![30^oC=[30+273]K=303K](https://img.qammunity.org/2020/formulas/chemistry/high-school/qgk25dipm0wmrera79tmilvyvp64mm2nlu.png)
R = Gas constant =
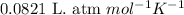
n = number of moles of hydrogen gas = ?
Putting values in above equation, we get:
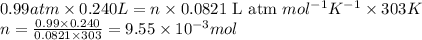
The chemical equation for the reaction of zinc and hydrochloric acid follows:
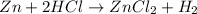
By Stoichiometry of the reaction:
1 mole of hydrogen gas is produced from 1 mole of zinc metal
So,
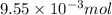 of hydrogen gas is produced from =
of hydrogen gas is produced from =
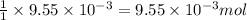 of zinc metal
of zinc metal
To calculate the mass of zinc metal, we use the equation:

Molar mass of zinc = 65.38 g/mol
Moles of zinc =
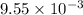 moles
moles
Putting values in above equation, we get:
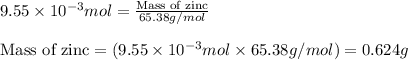
Hence, the mass or zinc reacted is 0.624 grams.