Step-by-step explanation:
Percentage w/v means that weight of solute present in 100 ml of a solution. Hence, 15% w/v means that 15 gram of solute per 100 ml of solution.
Hence, for 2 L (or 2000 mL) preparation the amount of mass will be calculated as follows.
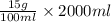
= 300 g
Thus, we can conclude that weight of Tris-HCL required is 300 g.