Step-by-step explanation:
The given data is as follows.
T = 298 K,
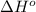 = -5645 kJ/mol
= -5645 kJ/mol
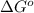 = -5798 kJ/mol
= -5798 kJ/mol
Relation between
 and
and
 are as follows.
are as follows.
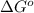 =
=
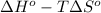
-5798 kJ/mol = -5645 kJ/mol -
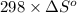
-153 kJ/mol = -
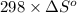
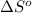 = 0.513 kJ/mol K
= 0.513 kJ/mol K
Now, temperature is
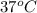 = (37 + 273) K = 310 K
= (37 + 273) K = 310 K
Since,
 =
=
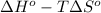
=
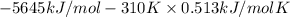
= (-5645 kJ/mol - 159.03 kJ/mol)
= -5804.03 kJ/mol
As, change in Gibb's free energy = maximum non-expansion work
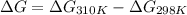
= -5804.03 kJ/mol - (-5798 kJ/mol)
= -6.03 kJ/mol
Therefore, we can conclude that the additional non-expansion work is -6.03 kJ/mol.