Answer:
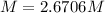
Step-by-step explanation:
Hello,
At first, the moles of magnesium sulfate must be computed by knowing that its molecular mass has a value of 120.366 g/mol:
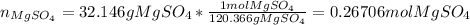
Now, by knowing that the volume must be in L, it turns out into 0.10000 L, thus:
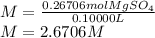
Best regards.