Answer:
The pH decreases.
Step-by-step explanation:
Hello,
In organic chemistry, oxidation accounts for either the increasing of C-O bonds or the increasing in the oxygen atoms into the molecule. Thus, if we consider the oxidation from benzyl alcohol to benzoic acid, there will be a carboxyl functional group instead of a hydroxile one. Now, the presence of the polar -O-H bonds that are ionizable, there will be a
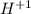 releasing causing the pH to decrease (increase acidity).
releasing causing the pH to decrease (increase acidity).
Regards.