Answer:
h = 9483 m
Step-by-step explanation:
Assume Re as radius of earth and h as equivalent thickness of co_2
total volume occupied by
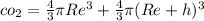
mass of
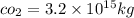
1 mole of co_2 has 44 g mass
1 g has = 1/44 mole
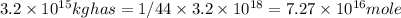
total volume by
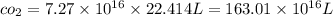
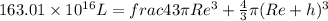
SOLVING RIGHT SIDE WE GET
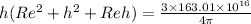
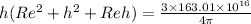
solving for h we get
h = 9483 m