Answer : The value of
 at this temperature is 66.7
at this temperature is 66.7
Explanation : Given,
Pressure of
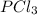 at equilibrium = 0.348 atm
at equilibrium = 0.348 atm
Pressure of
 at equilibrium = 0.441 atm
at equilibrium = 0.441 atm
Pressure of
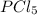 at equilibrium = 10.24 atm
at equilibrium = 10.24 atm
The balanced equilibrium reaction is,
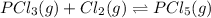
The expression of equilibrium constant
 for the reaction will be:
for the reaction will be:
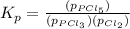
Now put all the values in this expression, we get :
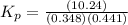
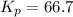
Therefore, the value of
 at this temperature is 66.7
at this temperature is 66.7