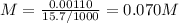Answer:
M(NaOH) = 0.07 M
Step-by-step explanation:
In a neutralization such as this the moles of the acid are the same as the base because you are titrating it until that occurs.
The moles of HCl can be obtained with the gas information using PV = nRT keep in mind to use consistent units.
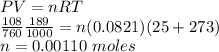
The molarity of NaOH:
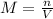
Because the moles of HCl are the same as NaOH