Answer:
49.7 mg of Ascorbic acid do not meet the daily requirement.
Step-by-step explanation:
The conversions are
Moles to mass, by multiplying with molar mass.
Molar mass is the mass of 1 mole of the substance.
Its unit is gram per mole g/mol
Ascorbic acid (
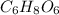 ) contains 6, C atoms 8, H atoms and 6, O atoms .
) contains 6, C atoms 8, H atoms and 6, O atoms .
We find the molar mass of the compound by just adding the atomic mass of the atoms present in it
Molar mass of Ascorbic acid
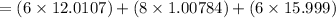
=176.124 g/mol(Given)
Mass of Ascorbic acid = Moles × Molar mass
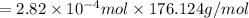
=0.0497 g
We know
1 g = 1000 mg
So
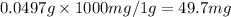 VITAMIN C
VITAMIN C
The required quantity for an Adult is 70 to 90mg.
So 49.7 mg of Ascorbic acid do not meet the daily requirement.(Answer)