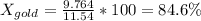Answer:
Percentage of gold = 84.6%
Step-by-step explanation:
Suppose the jewelry has x g of gold and y g of silver
The mass of both is the total mass of the jewelry then:
x + y = 11.54
And as the problem says the total volume is the sum of both volumes.
volume = mass / density
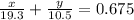
Solving the system you get:
x = 9.764 g
y = 1.776 g
The percentage of gold in the jewelry is: