Answer:
Energy conservation.
Step-by-step explanation:
The 1st Law of Thermodynamics is a statement about energy conservation. It states that
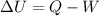 , which means that if we substract the work W done by the system to the heat Q given to the system we get the change in the internal energy
, which means that if we substract the work W done by the system to the heat Q given to the system we get the change in the internal energy
 , so any excess in energy given to the system appears as internal energy, stating that energy is conserved.
, so any excess in energy given to the system appears as internal energy, stating that energy is conserved.