Answer:
16064 m³/h
Option a) " Temperatures above about 0°C and pressures below about 1 atm "
Step-by-step explanation:
Given:
Temperature of oxygen = -65° C = 273 - 65 = 208 K
Pressure = 8.3 atm = 8.3 × 101325 Pa
Mass flow rate = 250 kg/h
also,
Molecular weight of oxygen, O₂ = 2 × 16 = 32 grams/mol = 0.032 kg/mol
now,
number of moles of oxygen, n =
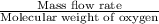
or
n =
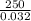
or
n = 7812.5 moles/h
Now,
from the ideal gas equation
PV = nRT
here,
V is the volume flow rate
P is the pressure
R is the ideal gas constant = 8.314 Pa.m³/mol.K
T is the temperature
thus,
V =
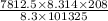
or
V = 16.064 m³/h
also,
1 m³ = 1000 L
thus,
V = 16.064 × 1000 m³/h = 16064 m³/h
Option a) " Temperatures above about 0°C and pressures below about 1 atm " is correct because the ideal gas law does not work at very low temperature and as well as at very high pressure