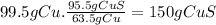Answer:
150 g of chalcocite (Cu) contain the same mass of Cu than 250 pounds of chalcopyrite.
Step-by-step explanation:
First, we need to pass 250 pound to the metric system using the relationship 1 lbs = 453g. Then,

Secondly, we look for the mass of copper in 1.13 x 10⁵ grams of CuFeS₂, knowing that there are 63.5g of Cu in 183.5 g of CuFeS₂ (molar masses).
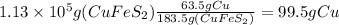
Finally, we find the mass of CuS that contains 99.5 g of Cu, knowing that 63.5 g of Cu are found in 95.5 g of CuS (molar masses).