Answer:
Step-by-step explanation:
In first place you use the molarity and volume of the diluted solution to determine how many moles of solute it contains. (The solute is the substance that dissolves in a solution to produce a homogeneous mixture.)
For that you should know that molarity or molar concentration is the number of moles of solute per liter of solution:
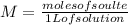
In this case, for the diluted solution, you have 0.1 M. This means that in 1 L of solution there are 0.1 moles. Then, to determine the amount of moles in 500 ml of solution, you make a rule of three, taking into account that 1 L is 1000 ml.
So: If 1000 ml of the solution has 0.1 moles, then how many moles are in 500 ml of solution?
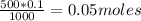
Now, in a concentrated and diluted solution, the solute number must be kept constant, that is, it does not change. What varies is its concentration, because the volume of solution varies.
With this in mind, you can determine how many milliliters would contain 0.05 moles of acid, taking into account the molarity of the concentrated HCl.
So: If 1000 ml of the solution has 12.1 moles, then how many milliliters of the reagent would contain 0.05 moles of HCl?
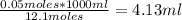
So, you need 4.13 ml of the reagent to make 0.100 M HCl.