Answer: 15.3 ml of water is to be added to 7.1 M solution.
Step-by-step explanation:
The relationship between specific gravity and density of a substance is given as:
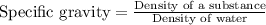
Specific gravity of
 = 1.18
= 1.18
Density of water =
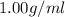
Putting values in above equation we get:
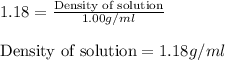
Given : 37 g of HCl is present in 100 g of solution
Volume of concentrated HCl solution=
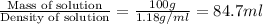
According to the dilution law,

where,
 = molarity of stock
= molarity of stock
 solution = ?
solution = ?
 = molarity of diluted
= molarity of diluted
 solution= 6.0 M
solution= 6.0 M
 = volume of stock
= volume of stock
 solution = 84.7 ml
solution = 84.7 ml
 = volume of diluted
= volume of diluted
 solution =
solution =
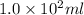
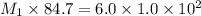
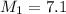
Therefore, the concentration of stock
 solution is 7.1 M
solution is 7.1 M
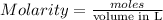
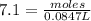
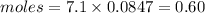
Mass of
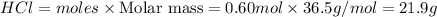
To prepare 7.1 M solution , we need to add 21.9 g of HCl to 84.7 ml of water.
To prepare
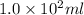 of 6.0 M HCl from a concentrated solution , (100-84.7) = 15.3 ml of water is to be added to 7.1 M solution.
of 6.0 M HCl from a concentrated solution , (100-84.7) = 15.3 ml of water is to be added to 7.1 M solution.