Answer:
isotope 1: 37.6%
isotope 2: 62.4%
Step-by-step explanation:
As the element has only two naturally occurring isotopes, we can name them as:
x = mass of isotope 2; so x = 47.000amu
(1-x) = mass of isotope 1; so (1-x) = 44.000amu
Then the average atomic mass of the element must be:
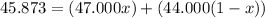
Solving for x, we have:
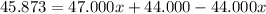
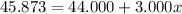
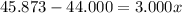
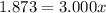
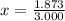
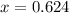
So, with x we can find (1-x):
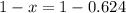
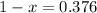
So, the abundance of isotope 1 is 37.6% and isotope 2 has an abundance of 62.4%.