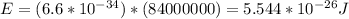Answer:
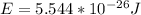
Explanation:
The equation for photon energy is given by:
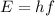
Where:
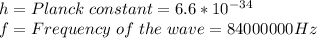
This equation telling us that the higher the frequency of the photon, the greater its energy. And the longer the photon wavelength, the lower its energy.
So, replacing the data provided in the previous equation: