Step-by-step explanation:
The given data is as follows.
coefficient of volume expansion of glycerin (
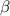 ) =
) =
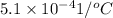
linear expansion coefficient of aluminum,
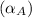 =
=
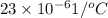
Volume = 100

The increase in volume of the cup will be calculated as follows.
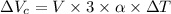
=
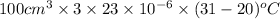
=
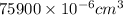
= 0.0759

Formula for increase in volume of glycerine is as follows.
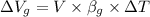
=
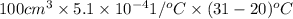
= 0.5610

Therefore, volume of glycerin spilled is calculated as follows.
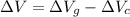
= (0.5610 - 0.0759)

= 0.4851

Thus, we can conclude that 0.4851
 glycerin will spill out of the cup.
glycerin will spill out of the cup.