Step-by-step explanation:
Molarity is defined as the number of moles of solute present in liter of solution.
Mathematically, Molarity =
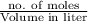
Also, when number of moles are equal in a solution then the formula will be as follows.
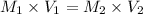
It is given that
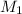 is 0.14 M,
is 0.14 M,
 is 0.083 L, and
is 0.083 L, and
 is 1.0 L.
is 1.0 L.
Hence, calculate the value of
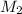 using above formula as follows.
using above formula as follows.
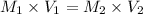
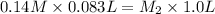
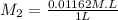
= 0.01162 M
Thus, we can conclude that the molarity of a solution is 0.01162 M.