Answer : The molecular weight of a substance is 157.3 g/mol
Explanation :
As we are given that 7 % by weight that means 7 grams of solute present in 100 grams of solution.
Mass of solute = 7 g
Mass of solution = 100 g
Mass of solvent = 100 - 7 = 93 g
Formula used :
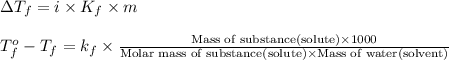
where,
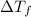 = change in freezing point
= change in freezing point
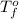 = temperature of pure water =
= temperature of pure water =
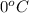
 = temperature of solution =
= temperature of solution =
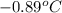
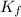 = freezing point constant of water =
= freezing point constant of water =
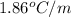
m = molality
Now put all the given values in this formula, we get
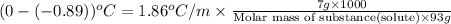
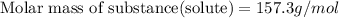
Therefore, the molecular weight of a substance is 157.3 g/mol