Answer:
The fraction of water body necessary to keep the temperature constant is 0,0051.
Step-by-step explanation:
Heat:
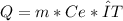
Q= heat (unknown)
m= mass (unknown)
Ce= especific heat (1 cal/g*°C)
ΔT= variation of temperature (2.75 °C)
Latent heat:
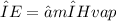
ΔE= latent heat
m= mass (unknown)
∝= mass fraction (unknown)
ΔHvap= enthalpy of vaporization (539.4 cal/g)
Since Q and E are equal, we can match both equations:
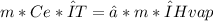
Mass fraction is:
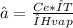
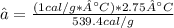
∝=0,0051