Answer:
A. H2O
Step-by-step explanation:
The balanced ionic equation for the reaction is:
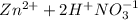 →
→
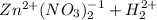
This implies that the products of the reaction are zinc nitrate and hydrogen gas only.
Therefore, the outlier from the options is water (H2O)
Let me know if you have any other questions.