Answer: Option (b) is the correct answer.
Step-by-step explanation:
A covalent bond is defined as the bond that is formed by sharing of electrons between the two chemically combining atoms.
For example, in a
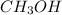 molecule.
molecule.
As the valency of each carbon atom is 4, valency of each hydrogen atom is 1, and valency of each oxygen atom is 2.
So, in
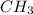 each one outermost electron of carbon atom is shared by each one outermost electron of hydrogen atom.
each one outermost electron of carbon atom is shared by each one outermost electron of hydrogen atom.
The fourth outermost electron is shared by one outermost electron of oxygen atom and the other outermost electron of oxygen atom is shared by the hydrogen atom of -OH.
Hence, we can conclude that there are total 5 covalent bonds present in the formation of methyl hydroxide ( or methanol).