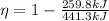Answer:
- The thermal efficiency is 0.4113.
Step-by-step explanation:
We know that the thermal efficiency is the ratio of work done by the engine over the heat taken
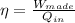
Now, how much work the engine do in a cycle?
We know that the work done in a cycle must be equal to the heat taken minus the heat rejected
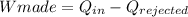
So, the thermal efficiency will be:
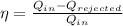
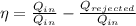
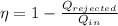
Putting the values of the problem