Answer:
The amount of energy needed when water at 72 degrees c freezes completely at 0 degrees c is
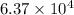 Joules
Joules
Step-by-step explanation:
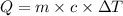
where
 = Final T - Initial T
= Final T - Initial T
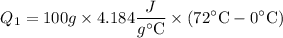
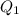 =30125J
=30125J
Q is the heat energy in Joules
c is the specific heat capacity (for water 1.0 cal/(g℃)) or 4.184 J/(g℃)
m is the mass of water
mass of water is assumed as 100 g (since not mentioned)
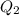 is the heat energy required for the phase change
is the heat energy required for the phase change
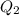 =mass × heat of fusion
=mass × heat of fusion
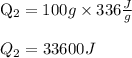
Total heat =
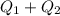
Total Heat = 30123J + 33600J
= 63725 J
=
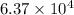 Joules is the answer
Joules is the answer