Answer: The correct answer is Option d.
Step-by-step explanation:
According to mole concept:
1 mole of a substance contains
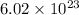 number of particles.
number of particles.
This number is known as Avogadro's number.
The mass of 1 mole of a substance or Avogadro number of particles has the mass equal to molar mass of that substance.
Hence, the correct answer is Option d.