Answer:
Option 2 Always is the Answer
Step-by-step explanation:
Breaking of bonds requires energy. Absorption of energy takes place to break a bond.
Formation of a bond results in the release of energy.
Hence breaking of bonds is an endothermic process and formation of a bond is exothermic.
The enthalpy of a reaction or the Heat energy of a reaction is given by
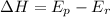
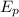 stands for energy of products
stands for energy of products
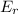 stands for energy of reactants
stands for energy of reactants
If
 then ∆H will be Positive and the reaction is Endothermic
then ∆H will be Positive and the reaction is Endothermic
If
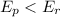 then ∆H will be Negative and the reaction is Exothermic
then ∆H will be Negative and the reaction is Exothermic