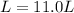A. 11.0 L is the researcher's volume measurement to the SL units of liter
Step-by-step explanation:
Given that a researcher recorded that the chemical reaction has water = 2.90 gallon.
We have to convert the researcher's volume into the SL units of liter.
So, we know that 1 gallon = 3.875 liters.
Then we have to convert 2.90 gallons of water in to liters.
Then , volume in L =
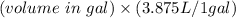 =
=
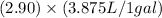
=
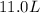
Then the required volume in