Let's divide the three experiments: The experiment with 10.00 mL of water is A), the experiment with 15.00 mL is B), and the experiment with 25.00 mL is C).
- (1) Now let's calculate the experimental density of each experiment. Density (ρ) is equal to the mass divided by the volume, thus:
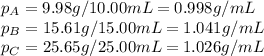
- (2)To calculate the average density, we add each density and divide the result by the number of experiments (in this case 3):
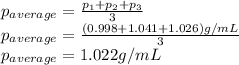
- (3) The percent error is calculated by dividing the absolute value of the substraction of the theorethical and experimental values, by the theoretical value, times 100:
%error=
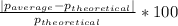
%error=
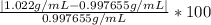
%error=2.44 %