Answer:
For 1: The percentage composition of
 in unknown carbonate sample is 31.79 %
in unknown carbonate sample is 31.79 %
For 2: The unknown carbonate sample is potassium carbonate.
Step-by-step explanation:
To calculate the percentage composition of carbon dioxide in sample, we use the equation:
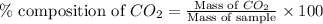 .....(1)
.....(1)
Mass of sample = 1.1000 g
Mass of carbon dioxide = 0.3497 g
Putting values in equation 1, we get:
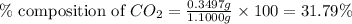
Hence, the percentage composition of
 in unknown carbonate sample is 31.79 %
in unknown carbonate sample is 31.79 %
We are given:
Mass of carbon dioxide =
![[1* 12.01)+(2* 16.00)]=44.01g](https://img.qammunity.org/2020/formulas/chemistry/college/h8m52p1e5z4nqtd7q8m084byj3kb5mw120.png)
Mass of lithium carbonate= 73.892 g
Mass of carbon dioxide = 44.01 g
Putting values in equation 1, we get:
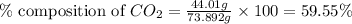
The percent composition of carbon dioxide in lithium carbonate is 59.55 %
Mass of potassium carbonate= 138.21 g
Mass of carbon dioxide = 44.01 g
Putting values in equation 1, we get:
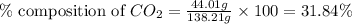
The percent composition of carbon dioxide in potassium carbonate is 31.84 %
Mass of sodium carbonate= 105.99 g
Mass of carbon dioxide = 44.01 g
Putting values in equation 1, we get:
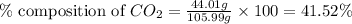
The percent composition of carbon dioxide in sodium carbonate is 41.52 %
Mass of calcium carbonate = 100.09 g
Mass of carbon dioxide = 44.01 g
Putting values in equation 1, we get:
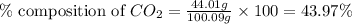
The percent composition of carbon dioxide in calcium carbonate is 43.97 %
As, the percent composition of carbon dioxide in the unknown sample matches the percent composition of carbon dioxide in potassium carbonate.
Hence, the unknown carbonate sample is potassium carbonate.