Answer:
Step-by-step explanation:
The mass percentages of chlorine and fluorine:
Given:
Mass of chlorine = 5.753g
Mass of fluorine = 9.248g
Find the total of the masses given:
Total mass = mass of chlorine + mass of fluorine = 5.753 + 9.248 = 15.001g
% of Cl =
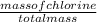 x 100
x 100
% of Cl =
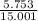 x 100 = 38.35%
x 100 = 38.35%
% of F =
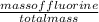 x 100
x 100
% of F =
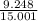 x 100 = 61.65%
x 100 = 61.65%