Answer:
The atomic weight of Rubidium (Rb) is 85.91 amu, using 4 significant figures.
Step-by-step explanation:
The average atomic mass of Rubidium (Rb) is composed by the mass of the two given isotopes 85^Rb and 87^Rb but each isotope has a different abundance in nature expressed by percentage (natural occurrence).
Therefore, we need to calculate how much of this mass with its abundance percentage contribute to the average atomic mass for each isotope.
First step: Calculate the contribution in mass using the natural occurrence percentage.
85^Rb = 84.91 ×
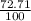 = 61.73 amu
= 61.73 amu
87^Rb = 86.91 ×
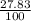 = 24.18 amu
= 24.18 amu
The answers for each isotope are given using a typical percentage formula.
Second step: Add the two mass values founded.
24.18 amu + 61.73 amu = 85.91 amu
Third Step: Check the significant figures
The answer is 85.91 amu and it has 4 significant figures.