Answer:
You need to do the following conversion to pass from 3M in 250 mL to g of sodium acetate
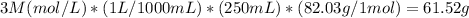
Step-by-step explanation:
First, you need to dissolve 61.52 g of solid sodium acetate (MW 82.03 g/mol) in 200 ml of DI water. Then, using a volumetric flask add water to bring the total volume of the solution to 250 mL.