Answer:
Step-by-step explanation:
Given
Pressure, Temperature, Volume of gases is
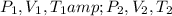
Let P & T be the final Pressure and Temperature
as it is rigid adiabatic container therefore Q=0 as heat loss by one gas is equal to heat gain by another gas
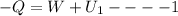
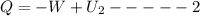
where Q=heat loss or gain (- heat loss,+heat gain)
W=work done by gas
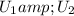 change in internal Energy of gas
change in internal Energy of gas
Thus from 1 & 2 we can say that
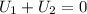
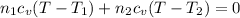
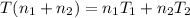
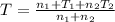
where
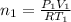
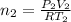
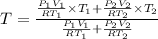
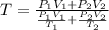
and
