Answer: The pH of resulting solution is 8.7
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
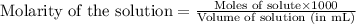
Molarity of TRIS acid solution = 0.1 M
Volume of solution = 50 mL
Putting values in above equation, we get:

Molarity of TRIS base solution = 0.2 M
Volume of solution = 60 mL
Putting values in above equation, we get:

Volume of solution = 50 + 60 = 110 mL = 0.11 L (Conversion factor: 1 L = 1000 mL)
- To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2020/formulas/chemistry/college/wwf6o5cvurukvvigp9qetx7pcmu718wast.png)
![pH=pK_a+\log(\frac{[\text{TRIS base}]}{[\text{TRIS acid}]})](https://img.qammunity.org/2020/formulas/chemistry/college/ntloknjspgp4wz5qqxywyizjfsigplyxdw.png)
We are given:
 = negative logarithm of acid dissociation constant of TRIS acid = 8.3
= negative logarithm of acid dissociation constant of TRIS acid = 8.3
![[\text{TRIS acid}]=(0.005)/(0.11)](https://img.qammunity.org/2020/formulas/chemistry/college/a7c9d5r17we042jc6doe5ovjwbh486nei3.png)
![[\text{TRIS base}]=(0.012)/(0.11)](https://img.qammunity.org/2020/formulas/chemistry/college/7nnqoj7pswsqehy13c7ug3wx900pi7lcek.png)
pH = ?
Putting values in above equation, we get:
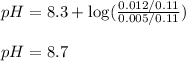
Hence, the pH of resulting solution is 8.7