Answer:
specific volume v = 0.0010385 m³/kg
and internal energy u = 416.23 KJ/kg
specific volume vf = 0.001043 m³/kg
and internal energy uf = 419.06 KJ/kg
% error in specific volume = 0.43 %
% error in internal energy = 0.679 %
Step-by-step explanation:
given data
pressure = 10MPa
temperature = 100 C
to find out
specific volume and internal energy and What percent error do you get
solution
we know pressure 10 MPa and temperature 100 C
so from compressed liquid water table we get
specific volume v = 0.0010385 m³/kg
and internal energy u = 416.23 KJ/kg
and
by using saturated liquid approx
from saturated water table of 100 C
specific volume vf = 0.001043 m³/kg
and internal energy uf = 419.06 KJ/kg
so
% error in specific volume is
% error in specific volume =
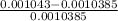 × 100
× 100
% error in specific volume = 0.43 %
and
% error in internal energy is
% error in internal energy =
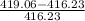 × 100
× 100
% error in internal energy = 0.679 %