Answer:
Specific change in internal energy is - 2.025 kj/kg.
Step-by-step explanation:
The process is constant pressure expansion. Apply first law of thermodynamic to calculate the change in internal energy.
Given:
Mass of gas is 4 kg.
Initial volume is 0.005 m³.
Final volume is 0.006 m³.
Pressure is 12 Mpa.
Heat is transfer to the gas. So it must be positive 3.9 kj.
Calculation:
Step1
Work of expansion is calculated as follows:
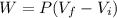
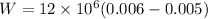
W=12000 j.
Or,
W=12 Kj.
Step2
Apply first Law of thermodynamic as follows:
Q=W+dU
3.9=12+dU
dU = - 8.1 kj.
Step3
Specific change in internal energy is calculated as follows:
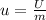
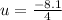
u= - 2.025 kj/kg.
Thus, the specific change in internal energy is - 2.025 kj/kg.