Answer:
Required compressor work W=8560.44 KJ/Kmol
Step-by-step explanation:
Given that
Initial pressure = 1 bar
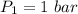
Final pressure = 40 bar
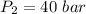
Process is isothermal and T=280 K
We know that ,work done in isothermal process given as
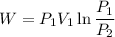
given taht gas is ideal so
P V =m R T
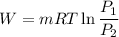
R for ethylene
R=0.296 KJ/kg.K
Now by putting the values
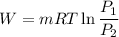
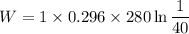
W= -305.73 KJ/kg
Negative sign indicates that work done on the system.
Required compressor work W=305.73 KJ/kg
Molar mass of ethylene M= 28 Kg/Kmol
So W= 305.73 x 28 KJ/Kmol
W=8560.44 KJ/Kmol
Required compressor work W=8560.44 KJ/Kmol