Answer : The mass of sodium fluoride added should be 0.105 grams.
Explanation : Given,
The dissociation constant for HF =
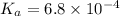
Concentration of HF (weak acid)= 0.0310 M
First we have to calculate the value of
 .
.
The expression used for the calculation of
 is,
is,
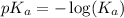
Now put the value of
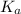 in this expression, we get:
in this expression, we get:
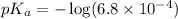
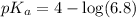
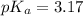
Now we have to calculate the concentration of NaF.
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2020/formulas/chemistry/college/6wyuhr9b7n0qwlgrnwgg688yylfbvv3wby.png)
![pH=pK_a+\log ([NaF])/([HF])](https://img.qammunity.org/2020/formulas/chemistry/college/a45iqo0m4vjk38hh5xj5qfilmr5mrzg9xc.png)
Now put all the given values in this expression, we get:
![2.60=3.17+\log (([NaF])/(0.0310))](https://img.qammunity.org/2020/formulas/chemistry/college/eqimzsi68khxte49gtern94lm4ha08g0jb.png)
![[NaF]=0.00834M](https://img.qammunity.org/2020/formulas/chemistry/college/bk2jtshzvhnhvfsqnmdz5uavjb7lydsypl.png)
Now we have to calculate the moles of NaF.
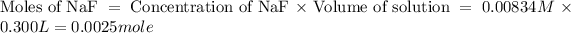
Now we have to calculate the mass of NaF.
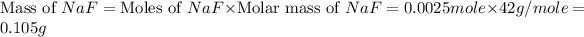
Therefore, the mass of sodium fluoride added should be 0.105 grams.