Answer: The mass of chlorine gas is 4.54 grams.
Step-by-step explanation:
To calculate the mass of the gas, we use the equation given by ideal gas equation:

Or,
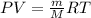
where,
P = pressure of the gas = 98.7 kPa
V = Volume of gas = 3.34 L
m = given mass of chlorine gas = ?
M = Molar mass of chlorine gas = 35.45 g/mol
R = Gas constant =
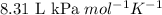
T = Temperature of the gas =
![37^oC=[37+273]=310K](https://img.qammunity.org/2020/formulas/chemistry/college/ng4m50k36ufwi9zo8jhbhm9g5iiml4peji.png)
Putting values in above equation, we get:
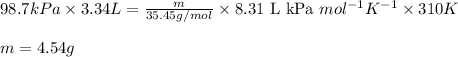
Hence, the mass of chlorine gas is 4.54 grams.