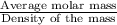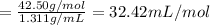Step-by-step explanation:
Mass of fructose = 33.56 g
Mass of water = 18.88 g
Total mass of the solution = Mass of fructose + Mass of water = M
M = 33.56 g + 18.88 g =52.44 g
Volume of the solution = V = 40.00 mL
Density =
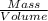
a) Density of the solution:
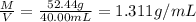
b) Molar mass of fructose = 180.16 g/mol
Moles of fructose =
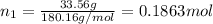
Molar mass of water = 18.02 g/mol
Moles of water=
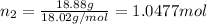
Mole fraction of fructose in this solution:
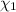
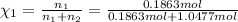
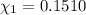
Mole fraction of water =
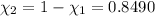
c) Average molar mass of of the solution:
=
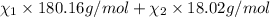
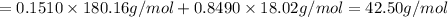
d) Mass of 1 mole of solution = 42.50 g/mol
Density of the solution = 1.311 g/mL
d) Specific molar volume of the solution: