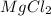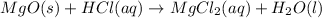Answer:
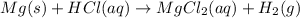
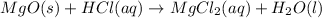
Step-by-step explanation:
When Mg reacts with HCl, magnesium chloride and hydrogen is formed. Mg is an active element and displaces hydrogen from HCl. So, this is a type of single displacement reaction.
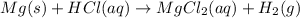
When magnesium oxide (MgO) reacts with HCl, magnesium chloride and water is formed. This reaction is a type of neutralization reaction. MgO is a water insoluble base and HCl is acid. So. in this reaction, acid reacts with base to form salt