Answer:
The rate of disappearance of
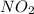 for this period is
for this period is

Step-by-step explanation:
Initial concentration of
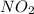 = x = 0.0138 M
= x = 0.0138 M
Final concentration of
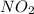 = y = 0.00886 M
= y = 0.00886 M
Time elapsed during change in concentration = Δt = 374 s
Change in concentration ,
![\Delta [NO_2]](https://img.qammunity.org/2020/formulas/chemistry/college/y8ygkrwqdaiwfuz4y4mfdp9qyhwvmca6g6.png) = y - x = 0.00886 - 0.0138 M = -0.00494 M
= y - x = 0.00886 - 0.0138 M = -0.00494 M
The rate of disappearance of
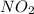 for this period is:
for this period is:
![(\Delta [NO_2])/(\Delta t)=(-0.00494 M)/(374 s)=-1.32* 10^(-5) M/s](https://img.qammunity.org/2020/formulas/chemistry/college/gclhrexvm1i7fp4mt7ujuoy4tzfcuzu1zt.png)