Answer:
You need to add 203 mL of 1,00M KH₂PO₄ and 147 mL of 1,00M K₂HPO₄.
Step-by-step explanation:
It is possible to use Henderson–Hasselbalch equation to estimate pH in a buffer solution:
pH = pka + log₁₀
Where A⁻ is conjugate base and HA is conjugate acid
The equilibrium of phosphate buffer is:
H₂PO₄⁻ ⇄ HPO4²⁻ + H⁺ Kₐ₂ = 6,20x10⁻⁸; pka=7,21
Thus, Henderson–Hasselbalch equation for 7,07 phosphate buffer is:
7,07 = 7,21 + log₁₀
![([HPO4^(2-)] )/([H2PO4^(-)])](https://img.qammunity.org/2020/formulas/chemistry/college/3casie8b7zjdxi9tomoxbnq7q7f15y8fwb.png)
0,7244 =
![([HPO4^(2-)] )/([H2PO4^(-)])](https://img.qammunity.org/2020/formulas/chemistry/college/3casie8b7zjdxi9tomoxbnq7q7f15y8fwb.png) (1)
(1)
As the buffer concentration must be 1,00 M:
1,00 = [H₂PO₄⁻] + [HPO4²] (2)
Replacing (2) in (1):
[H₂PO₄⁻] = 0,5799 M
Thus:
[HPO4²] = 0,4201 M
To obtain these concentrations you need to add:
0,5799 M × 0,350 L ×
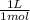 = 0,203 L ≡ 203 mL of 1,00M KH₂PO₄
= 0,203 L ≡ 203 mL of 1,00M KH₂PO₄
And:
0,4201 M × 0,350 L ×
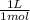 = 0,203 L ≡ 147 mL of 1,00M K₂HPO₄
= 0,203 L ≡ 147 mL of 1,00M K₂HPO₄
I hope it helps!