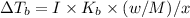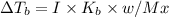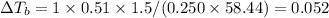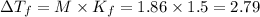Answer:
 for given question is 2.79 and
for given question is 2.79 and
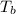 is 0.52
is 0.52
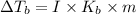 {i- vant hoff’s constant ; Kb- constant ; m molarity }
{i- vant hoff’s constant ; Kb- constant ; m molarity }
M = no. of moles of the solute present in one kg of solution
Let the weight of amount of solute be “w” and its molecular mass be “M”
Let the mass of the solvent in the given question be “x”