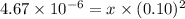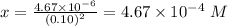Answer:
Molar solubility of [Pb]^{2+}][Br^-]^2[/tex] =
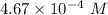
Step-by-step explanation:
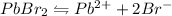
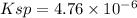
Let the molar solubility of
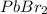 be x
be x
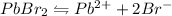
At equi. x 2x
![[Pb]^(2+)](https://img.qammunity.org/2020/formulas/chemistry/college/oqqvn90p9hln6ksma3niwrj5tk2xbnsppe.png) = x
= x
![[Br]^(-)](https://img.qammunity.org/2020/formulas/chemistry/college/5hfmt77v4sk5bhbzmcp8ut6awqz53djl6i.png) = 2x
= 2x
In the presence of 0.10 M NaBr
![[Br]^(-)](https://img.qammunity.org/2020/formulas/chemistry/college/5hfmt77v4sk5bhbzmcp8ut6awqz53djl6i.png) = 2x + 0.10
= 2x + 0.10
![Ksp = [Pb]^(2+)][Br^-]^2](https://img.qammunity.org/2020/formulas/chemistry/college/8s3y896kfvjntyoh7szgn963pg56u8q2uc.png)
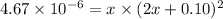
As
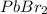 is weakly soluble, so 2x<<0.1.
is weakly soluble, so 2x<<0.1.
2x can be neglected as compared to 0.1