Answer: A) The reaction is spontaneous above 276 K.
Step-by-step explanation:
According to Gibb's equation:
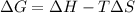
 = Gibbs free energy
= Gibbs free energy
 = enthalpy change = +29.3 kJ/mol =29300 J/mol
= enthalpy change = +29.3 kJ/mol =29300 J/mol
 = entropy change = +106 J/molK
= entropy change = +106 J/molK
T = temperature in Kelvin
 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 = -ve, reaction is spontaneous
= -ve, reaction is spontaneous
 = 0, reaction is in equilibrium
= 0, reaction is in equilibrium
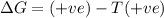
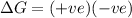
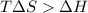 for reaction to be spontaneous
for reaction to be spontaneous
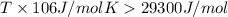
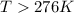
Thus the Reaction is spontaneous when temperature is above 276 K.